|
Radioactive isotopes decay spontaneously; that is, the decay reaction occurs randomly without outside influences. If we look at the nucleus of a radioactive isotope, we do not know when it will undergo radioactive decay. It could be in the next second, a few minutes from now, or in days, years, centuries—or even in a few millennia. In the macroscopic world, the forces of gravity and electromagnetism are deterministic, meaning that we can write down the equations and can calculate exactly how every object moves and interacts with the environment. In the microscopic world of the nucleus, however, the weak force and the decay of radioactive atoms is statistical, not deterministic. This is one way in which our everyday world and the quantum world are fundamentally different. 
|
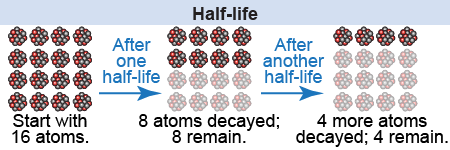 The number of atoms that will decay radioactively in a fixed period of time is determined by the isotope’s half-life. In the time period of one half-life, half of those radioactive atoms will undergo radioactive decay. The half-life is denoted by the symbol t½. Imagine that you have 16 atoms of the same radioactive isotope and it has a half-life of one hour. After one hour, half of those atoms—eight of them—will have decayed, while the other eight are unchanged. Now wait another hour. Of the eight previously undecayed atoms, half of them—four more atoms—will decay in this second hour, while the other four atoms are still unchanged. Wait again for an hour. At the end of this third hour, half of the four undecayed atoms—two more atoms—will have decayed, while the other two still stay the same. That is how half-life works: After the time period of one half-life, half of the atoms will have decayed radioactively.
The number of atoms that will decay radioactively in a fixed period of time is determined by the isotope’s half-life. In the time period of one half-life, half of those radioactive atoms will undergo radioactive decay. The half-life is denoted by the symbol t½. Imagine that you have 16 atoms of the same radioactive isotope and it has a half-life of one hour. After one hour, half of those atoms—eight of them—will have decayed, while the other eight are unchanged. Now wait another hour. Of the eight previously undecayed atoms, half of them—four more atoms—will decay in this second hour, while the other four atoms are still unchanged. Wait again for an hour. At the end of this third hour, half of the four undecayed atoms—two more atoms—will have decayed, while the other two still stay the same. That is how half-life works: After the time period of one half-life, half of the atoms will have decayed radioactively. 
 |
At the beginning of that process, you knew that statistically half of the original 16 atoms would decay by the end of the first hour. But could you have predicted which atoms would decay and which would not? Not at all! You cannot predict which ones will decay—only that half of them will. That is the craziest thing about statistical processes in the quantum world. Even Albert Einstein, a genius among physicists, showed great reluctance in believing the equations of statistics in the quantum world, contending that, “God doesn’t play dice with the world.” For once, was Einstein wrong? 
|
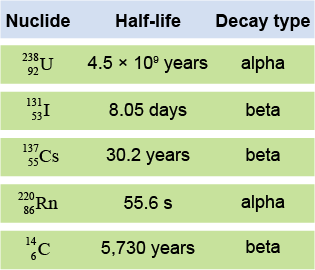 Some radioactive isotopes have a very short half-life, whereas others have a half-life of billions of years. For example, uranium-238 has a half-life of 4.5 billion years and so it has been around since the formation of our Solar System. Iodine-131 and cesium-137 are radioactive products of nuclear fission. Fluorine-18, which is a isotope used in positron emission tomography (PET), has a half-life of about 110 minutes and so it does not occur naturally. Isotopes with very short half-life are generated in the laboratory.
Some radioactive isotopes have a very short half-life, whereas others have a half-life of billions of years. For example, uranium-238 has a half-life of 4.5 billion years and so it has been around since the formation of our Solar System. Iodine-131 and cesium-137 are radioactive products of nuclear fission. Fluorine-18, which is a isotope used in positron emission tomography (PET), has a half-life of about 110 minutes and so it does not occur naturally. Isotopes with very short half-life are generated in the laboratory. 
|
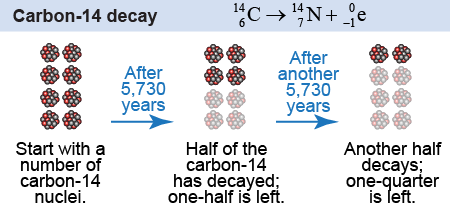 Every half-life, the number of radioactive nuclei decreases by half. The number of carbon-14 nuclei in a sample will decrease by half every 5,730 years. As carbon-14 decays via beta decay, nitrogen-14 is generated.
Every half-life, the number of radioactive nuclei decreases by half. The number of carbon-14 nuclei in a sample will decrease by half every 5,730 years. As carbon-14 decays via beta decay, nitrogen-14 is generated. 
|
|
|
After a tree dies, the carbon-14 in it stops being replenished and starts decaying. After 11,460 years, what percentage of carbon-14 is left? - 12.5%
- 25%
- 50%
- 100%
 |
The answer is b, 25%. 11,460 years is two half-lives. After the first half life, 50% is left, and after the second, 25% is left. 
|
| |
|

