|
The strong nuclear force has a number of important properties that determine its effect on atomic particles. The strong nuclear force - is only attractive, not repulsive;
- acts only on nuclear particles or the particles that make up nucleons, not electrons;
- acts on charged particles (protons) and neutral particles (neutrons); and
- is a short-range force.

|
The strong nuclear force attracts every proton or neutron to every other proton or neutron regardless of electric charge. Both protons and neutrons are classed as nucleons, and the strong force attracts all nucleons to all nearby nucleons. The strong force does not affect electrons just as the electric force does not affect neutrons. 
|
The strong force is peculiar in that it acts only when particles are very close to each other, such as within an atomic nucleus. For this reason the strong nuclear force is called a short-range force. Although the strong nuclear force is very powerful within the nucleus, its short range means that it is unimportant beyond the scale of the nucleus. By comparison, the electromagnetic force is a long-range force. Two charges can exert an electromagnetic force on each other at any distance. The electric force gets weaker as 1/r2 but the strong force effectively drops to zero at distances much larger than 10−15 m! 
|
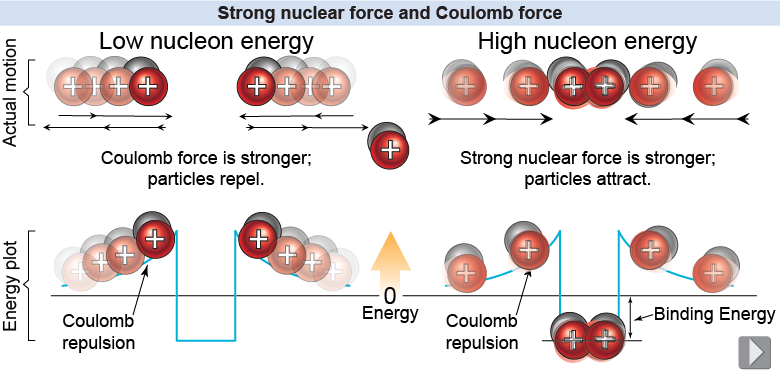
To get a sense of what “short range” means, consider two deuterium nuclei moving toward each other. A deuterium nucleus consists of a proton and a neutron. If the kinetic energy is low, the nuclei repel each other because the Coulomb force pushes them back apart before they get close enough for the strong nuclear force to take hold. At higher kinetic energies, if the separation becomes smaller than about 10−15 m, the strong nuclear force snaps them together and they become an alpha particle (helium nucleus). 
|
|
It takes energy to separate nucleons once they are bound together by the strong force. This energy is called the binding energy. Nuclear binding energy is enormous. For example, to separate the protons and neutrons in 1 kg of water (the amount in a 1 L bottle) would take 1013 J! This is the equivalent energy of 100,000 gallons of gasoline, all derived from the nuclear binding energy in just one liter of water. 
|
What subatomic particles are affected by the strong nuclear force? - protons and neutrons only
- protons and electrons only
- neutrons and electrons only
- protons, neutrons, and electrons
 |
The correct answer is a. The strong nuclear force affects particles in the nucleus—nucleons, which are protons and neutrons. 
|

