|
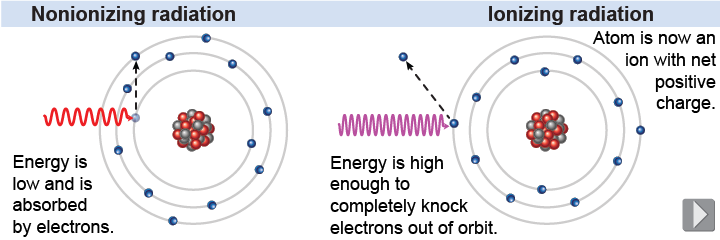
With sufficient energy, an electron can be removed from its orbit, leaving the atom positively charged or ionized. As we learned in Chapter 26, the amount of energy required to completely remove an electron from its orbit varies but it roughly falls in the range between 5 and 20 eV. This is a small amount of energy, but the effects that it has on the chemical and biological properties of the elements and molecules are significant. Ionization is associated with the emission of radiation. 
|
|
The normal functions of biological systems depend on the various chemical compounds that make up the system. Chemical bonds of molecules such as DNA depend on the exchange or sharing of electrons between and among the various elements that make up the molecules. When a biological system is exposed to radiation and the energy of the radiation is sufficient to result in ionization, the chemical bonds are affected. When a bond is broken or changed in any way, the properties and the function of the associated molecule may also change. Biological systems are able to correct the damage caused by small amounts of radiation. As the radiation increases, however, the damage to the molecules and proteins causes permanent damage such as genetic defects and cancer. 
|
The energy associated with radiation is used to detect it. When radiation interacts with matter it deposits energy in it, resulting in a number of changes at the atomic and molecular level. Radiation detection devices such as the Geiger counter detect these changes and use electronic processing to present the result as an audible popping sound. Radiation detectors are used for maintaining a safe working environment around radiation sources and to search for a potential radiation leak. 
|
Humans who remain close to radioactive substances experience a total exposure to radiation over time called the dose. The unit of measurement for exposure to radioactive or ionizing radiation is called the rem. A rem is a large dose of radiation and so exposures are usually estimated in millirem, or one-thousandth of a rem. 
|
Although large doses of radioactivity can be harmful to the human body, small targeted doses of radiation are also used as a medical therapy to kill cancer cells in the body. In nuclear medicine, radiation therapy (or radiotherapy) is a process of targeting radiation toward a part of the body that has many cancer cells in order to kill them. Some cancers, such as leukemia, respond well to radiotherapy, but others do not. The dose of radiation is chosen to balance the benefits of killing cancerous cells with the risks of damaging healthy cells. 
|
| |
|

