|
 Radioactivity describes the property of certain nuclei to emit energy. The word radioactivity was coined by Polish-born French chemist and physicist Marie Curie, who was the first to systematically investigate the phenomenon. She was the first woman to win a Nobel prize and the only woman ever to win two Nobel prizes (one each in physics and chemistry). Radioactivity carries energy derived from the structure of the nucleus. The energy associated with radioactivity can be hazardous to biological systems since it can penetrate tissues and deposit energy, thereby damaging cells and vital organs. Radioactivity, however, may also be used as a diagnostic and treatment tool, again by virtue of its ability to penetrate matter.
Radioactivity describes the property of certain nuclei to emit energy. The word radioactivity was coined by Polish-born French chemist and physicist Marie Curie, who was the first to systematically investigate the phenomenon. She was the first woman to win a Nobel prize and the only woman ever to win two Nobel prizes (one each in physics and chemistry). Radioactivity carries energy derived from the structure of the nucleus. The energy associated with radioactivity can be hazardous to biological systems since it can penetrate tissues and deposit energy, thereby damaging cells and vital organs. Radioactivity, however, may also be used as a diagnostic and treatment tool, again by virtue of its ability to penetrate matter. 
|
Radioactive decay
|
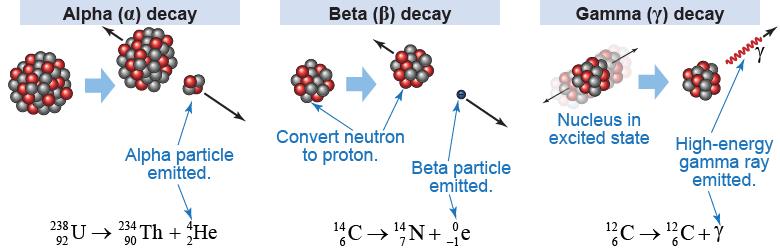
Radioactivity is released by nuclear processes known as radioactive decay. A radioactive decay is a process by which a nucleus spontaneously undergoes a change that emits energy. The three most common forms of radioactive decay are denoted by the first three letters of the Greek alphabet: alpha (α), beta (β), and gamma (γ) decay. Alpha and beta decay result in the emission of particles from the nucleus; gamma decay is the release of a high-energy photon. As a result, alpha and beta decay change the atomic number of the decaying atom, but gamma decay does not. 
|
|
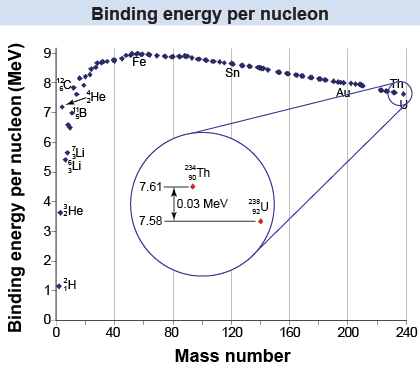 When a nucleus decays, it changes from a state with less binding energy to a state with more binding energy. The change in binding energy is both the cause of the change and the source of the emitted energy. For example, when uranium-238 decays via alpha decay it becomes thorium-234. The binding energy per nucleon in the nucleus is 7.58 MeV. For , the binding energy per nucleon is 7.61 MeV. The difference in the binding energy of the two nuclei (7.61 − 7.58) MeV = 0.03 MeV is the energy associated with the released alpha particle. Note that is more stable because the binding energy is higher.
When a nucleus decays, it changes from a state with less binding energy to a state with more binding energy. The change in binding energy is both the cause of the change and the source of the emitted energy. For example, when uranium-238 decays via alpha decay it becomes thorium-234. The binding energy per nucleon in the nucleus is 7.58 MeV. For , the binding energy per nucleon is 7.61 MeV. The difference in the binding energy of the two nuclei (7.61 − 7.58) MeV = 0.03 MeV is the energy associated with the released alpha particle. Note that is more stable because the binding energy is higher. 
|
Which type of radioactive decay does not change the atomic number of the decaying atom? - alpha decay
- beta decay
- gamma decay
 |
The answer is c, gamma decay. In gamma decay, the atom emits a gamma ray, but doesn’t change atomic number. 
|
| |
|

