|
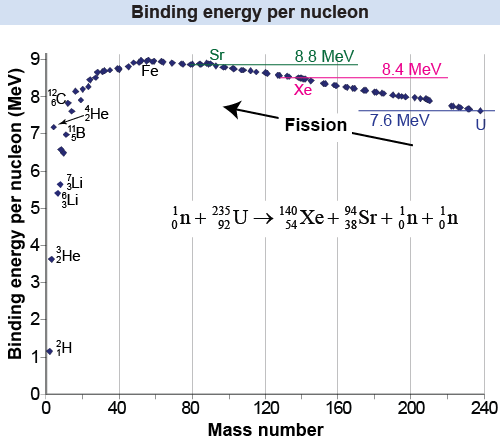 A nuclear reaction derives its energy from the binding energy of the nucleus. If a large nucleus such as uranium is broken apart, energy can be released. Splitting a nucleus is called a nuclear fission reaction. An example is the nuclear reaction that splits the nucleus into two products, strontium-94, and xenon-140. The energy released by this reaction can be estimated with an energy balance calculation of the binding energy.
A nuclear reaction derives its energy from the binding energy of the nucleus. If a large nucleus such as uranium is broken apart, energy can be released. Splitting a nucleus is called a nuclear fission reaction. An example is the nuclear reaction that splits the nucleus into two products, strontium-94, and xenon-140. The energy released by this reaction can be estimated with an energy balance calculation of the binding energy. 
|
From the binding energy plot we see that the binding energy per nucleon for uranium, xenon, and strontium are approximately 7.6, 8.4, and 8.8 MeV, respectively. The energy released is then approximately equal to This is very close to the actual energy release, which is 208 MeV. 
|
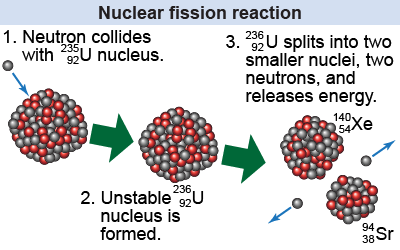
| What initiates a fission reaction? In the late 1930s, German physicist Lise Meitner and chemists Otto Hahn and Fritz Strassmann discovered that bombarding uranium atoms with neutrons can initiate a fission reaction. The incoming neutron is initially absorbed into the nucleus, turning it into an unstable nucleus. The nucleus then undergoes decay into a pair of nuclei, ejecting two neutrons. |

|
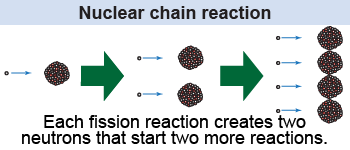 Bombarding a atom with one neutron can cause a nuclear fission reaction that ejects two neutrons. If there are many uranium atoms nearby, then those two ejected neutrons can initiate two new fission reactions that eject a total of four neutrons. Those four neutrons can start four new fission reactions that produce eight neutrons, and so on. This process is called a chain reaction and it is the key to continuous nuclear energy production.
Bombarding a atom with one neutron can cause a nuclear fission reaction that ejects two neutrons. If there are many uranium atoms nearby, then those two ejected neutrons can initiate two new fission reactions that eject a total of four neutrons. Those four neutrons can start four new fission reactions that produce eight neutrons, and so on. This process is called a chain reaction and it is the key to continuous nuclear energy production. 
|
Which of the following atoms might act as fuel for a nuclear fission reactor? - hydrogen
- helium
- carbon
- plutonium
 |
The answer is d, plutonium. When plutonium fissions it produces lighter nuclei with an overall binding energy balance similar to the fission of uranium. 
|

