|
Challenge: Create a lemon battery with a voltage exceeding 2 V.
Constraints: Use up to five lemons, as many nails and wires as you need, and any metal objects you have.
Performance evaluation: The measured voltage must be V ≥ 2 V, as measured by a voltmeter or digital multimeter. 
|
A simple lemon and two metal objects can create a single-cell battery. Both a store-bought alkaline battery and a lemon battery rely on chemical reactions that cause positive charges to move toward one metal and negative charges to move toward the other. If you insert two appropriate metal objects (called electrodes) into the lemon, the chemical reaction creates a voltage between the two metals. 
|
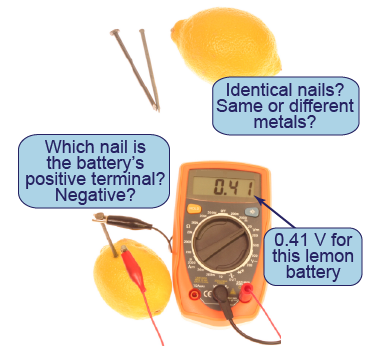 What parameters are important in creating a lemon cell and battery?
What parameters are important in creating a lemon cell and battery? - Try inserting two identical nails and measure the voltage between them. Then try two nails made from different metals. Record the voltage in each case.
- Insert two nails close to each other and then far apart. Measure the voltage for each case.
- Try to create a two-cell battery from your lemon. Try different kinds of nails and locations for the nails.

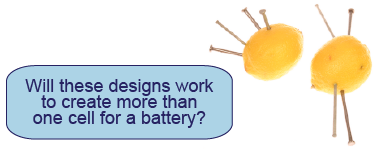 Based on what you discover, design a lemon battery that consists of more than one cell and that will create a voltage of at least 2 V. Write down your design using a combination of text and diagrams. Make a prediction for your design’s voltage, justify your prediction, and write down a procedure for testing and evaluating it.
Based on what you discover, design a lemon battery that consists of more than one cell and that will create a voltage of at least 2 V. Write down your design using a combination of text and diagrams. Make a prediction for your design’s voltage, justify your prediction, and write down a procedure for testing and evaluating it. 
|
Construct the prototype of your lemon battery design using the equipment. 
|
Use a voltmeter or digital multimeter to measure the voltage across your prototype lemon battery. Also measure the voltage across each individual cell in the battery. What were the variables you tested? 
|
How well did the performance of your prototype match your prediction? Evaluate and document the strengths and weaknesses of your prototype’s performance compared to the 2 V design criterion. 
|
Evaluate the individual elements in your design and determine how to improve it. Create a revised design, prototype it, and test it. If you succeed in creating a 2 V battery, then try to create even higher voltage from the materials! 
| |
| |
|

