|
Electric current is the flow of electric charge through a material such as a conducting wire. Electric current flows in response to differences in electrical potential, or voltage. A battery uses chemical potential energy to generate a voltage, which can cause electric current to flow in a conductor. A closed circuit provides at least one complete conducting path for current to travel in a closed loop. An open circuit, such as a flashlight that has been turned off, contains a break in the conducting path and so does not enable current to flow. Circuits must contain a voltage source and can also contain components such as light bulbs, resistors, and switches. 
|
|
electricity, electric current, ampere (A), electric circuit, open circuit, closed circuit, electrical symbol, short circuit, volt (V), potential difference, voltage, voltmeter, battery
|
Review problems and questions |
|
- One mole of electrons is approximately 6.0×1023 electrons (that’s six hundred billion trillion electrons). The ampere is the SI unit of electric current, but we could also measure current in moles of electrons per second. How many amperes equal one mole of electrons per second?

 |
Answer: One mole of electrons is roughly 9.7×104 A.
To establish the relationship between amperes (A) and moles per second (mol/s), we use the number of electrons per second in one amp (listed on page 472). It is a matter of canceling units to convert from mol/s into A: One mole of electrons per second corresponds to a current of approximately 97,000 A. That is a much, much larger amount of current than you will encounter in everyday life. 
|
- Portable electronic devices such as cellphones and media players have rechargeable batteries. When a battery is fully charged this means that it contains its maximum energy, which it can then supply to the electric charges that pass through it. The energy a battery provides is often stated in units of milliampere-hours, or mAh (based on its voltage). Suppose that your media player is rated at 100 mAh.
- How many electrons does 100 mAh represent?
- Suppose that you can listen to 5 hr of continuous music on this device after fully charging its battery. What is the average current in milliamperes (mA) that flows through the device while you listen to it?

 |
Answer: - 100 mAh represents approximately 2.2×1021 electrons.
- The average current is 20 mA.
Solution: - 100 mAh represents approximately 2.2×1021 electrons. Since current equals a certain number of electrons per second, we can multiply amperes by seconds to get electrons. Let’s do this in steps. First, let’s convert milliamperes into amperes. Remember that milli- means one one-thousandth (or 0.001) of something: so Next, we need to express amperes in terms of electrons per second: or Third, we figure out how many seconds are in one hour: We now know how many electrons are delivered each second, and how many seconds there are in one hour. Electrons per second multiplied by seconds per hour equals the number of electrons moving hourly: The quantity 100 mAh is equivalent to the charge on 2.2×1021 electrons.
- If the battery has just enough energy to provide a current of 100 mA for 1 hr, then it can also provide a current of 20 mA for 5 hr.

|
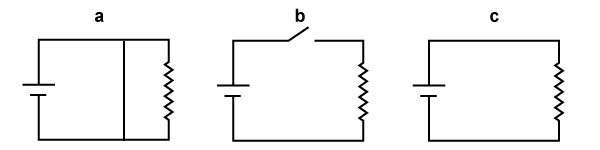
- Identify each of the circuits shown above as a closed circuit, an open circuit, or a short circuit. Each term is used only once.

 |
a = short circuit, b = open circuit, and c = closed circuit. A short circuit allows current to flow from one battery terminal to the other without encountering any significant resistance. An open circuit does not allow current to flow from one battery terminal to the other in any way whatsoever. A closed circuit allows electric current to flow from one battery terminal to the other, passing through a resistive component along the way. (Note that a short circuit is also a closed circuit—but not all closed circuits are short circuits!) 
|
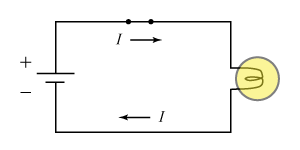
- This circuit diagram for a simple flashlight shows the direction of current flow, as conventionally defined. It also shows the positive and negative terminals of the battery. What is the relationship between electron flow and the arrowed current shown here?

 |
Electron flow is opposite the arrowed current. The particles that actually flow in a current-carrying metal wire are electrons. Electrons are negatively charged, so they must emerge from the battery’s negative (−) terminal. In the diagram shown, electrons will circulate in a counterclockwise fashion, through the wires, bulb, and switch, and on to the positive (+) terminal. Conventional current, by contrast, originates with the positive terminal and flows to the negative one. Therefore the arrowed current is opposite the direction of electron flow. 
|
- A student constructed a one-lamp circuit placing both ends of the lamp socket into the middle holes of row 11 of a circuit breadboard. He then connected a battery to the circuit using wires placed into holes on the two ends of row 11. Will his circuit work? Why or why not?

|
No, because he has shorted out both ends of the lamp—as well as both ends of the battery! The problem is that the student connected both leads of the lamp socket into the same row of the circuit breadboard, which causes both leads to be connected to each other. The student should instead place one lead from the lamp socket into one row of the breadboard and the other lead into a different row. 
|
- A student has two batteries to dispose of, one alkaline and the other NiCd.
- Are either or both of the batteries considered hazardous waste?
- What is the procedure for disposing of each?

|
- The NiCd rechargeable battery is considered hazardous waste, while the alkaline battery is not.
- Alkaline batteries can be disposed of in the standard waste stream (garbage can). Nickel–cadmium batteries must go to a waste management center or hardware store.

|

