|
A battery converts stored chemical energy into electrical power. The amount of power per ampere of current depends on the voltage of the battery. One volt means one watt of power for each ampere of current. For example, 1 A of current flowing from a 1.5 V battery delivers 1.5 W of electrical power. 
|
A battery stores a fixed amount of energy, which is depleted as current flows. For example, an AA size (1.5 V) alkaline battery stores 10,000 J of energy. A current of 1 A will deliver 1.5 J/s (1.5 W). At this rate the battery’s energy is depleted in under 2 hours. A lower current depletes the energy more slowly and the battery lasts longer. A larger “D” battery has the same voltage (1.5 V) but contains more chemicals and therefore has more stored energy. An alkaline “D” battery might contain 70,000 J of stored energy compared to only 5,000 J for a “AAA” size. 
|
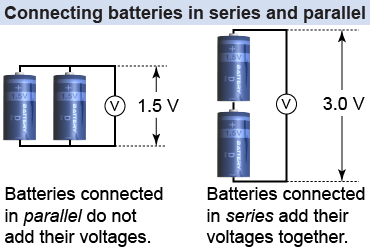 Batteries can be connected in two ways. When they are connected in parallel—positive terminals together and negative terminals together—the combination has the same voltage as a single battery but can produce twice the current. When the batteries are connected end to end, or in series, the combination has twice the voltage but provides the same current as a single battery.
Batteries can be connected in two ways. When they are connected in parallel—positive terminals together and negative terminals together—the combination has the same voltage as a single battery but can produce twice the current. When the batteries are connected end to end, or in series, the combination has twice the voltage but provides the same current as a single battery. 
|
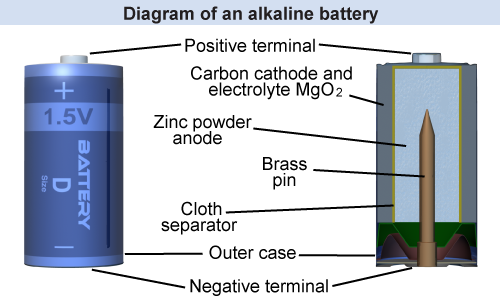 Inside a typical battery, the positive terminal is one kind of metal and the negative terminal is a different kind of metal. Both metals are immersed in an electrolyte, a material that undergoes a chemical reaction. The reaction releases electrons that are repelled from the negative terminal, forming the electric current.
Inside a typical battery, the positive terminal is one kind of metal and the negative terminal is a different kind of metal. Both metals are immersed in an electrolyte, a material that undergoes a chemical reaction. The reaction releases electrons that are repelled from the negative terminal, forming the electric current. 
|
Alkaline batteries use MgO2 (manganese dioxide) mixed with carbon for the cathode, zinc (in powder form) for the anode, and potassium hydroxide as the electrolyte. A porous membrane separates the cathode and anode. A fresh alkaline battery produces a voltage of about 1.62 V and is guaranteed to maintain a voltage of at least 1.5 V for a certain amount of current and time. For example, a 1.5 V AA-size alkaline battery can supply a current of about 1 A for about 50 minutes before its voltage drops below 1.5 V. Rechargeable nickel–cadmium (NiCd) batteries have a voltage of 1.2 V. Lead acid batteries are used in car engines and usually have a voltage of 12 V. 
|
Dispose of batteries properly. Most alkaline batteries today no longer contain mercury and can be put in the trash. Rechargeable batteries—such as NiCd or car batteries—must be recycled at a waste management center or through an auto dealer or hardware store. 
|
A battery converts _______ energy to electrical energy. - nuclear
- chemical
- thermal
- mechanical
 |
The correct answer is b, chemical. Inside a battery, a mixture of chemicals creates a potential difference between the two terminals, so that electricity will flow. 
|

