|
The richness of nuclear phenomena from radioactivity to nuclear energy generation is derived from the balance between the attractive and repulsive forces among the nucleons. The attractive nuclear force is experienced by both protons and neutrons, whereas the repulsive Coulomb force is experienced only by the positively charged protons. 
|
For all elements heavier than hydrogen, the balance of forces in the nucleus depends strongly on the number of neutrons. The “neutron glue” that holds the nucleus together comes from the action of the strong nuclear force. Without enough neutrons, the overall effect of the strong nuclear force is not sufficient to hold the nucleus together against the Coulomb force. If there are enough neutrons, then the attraction from the strong nuclear force overcomes the Coulomb force and the nucleus stays together. To achieve this force balance, every element heavier than helium has least one neutron for every proton. 
|
|
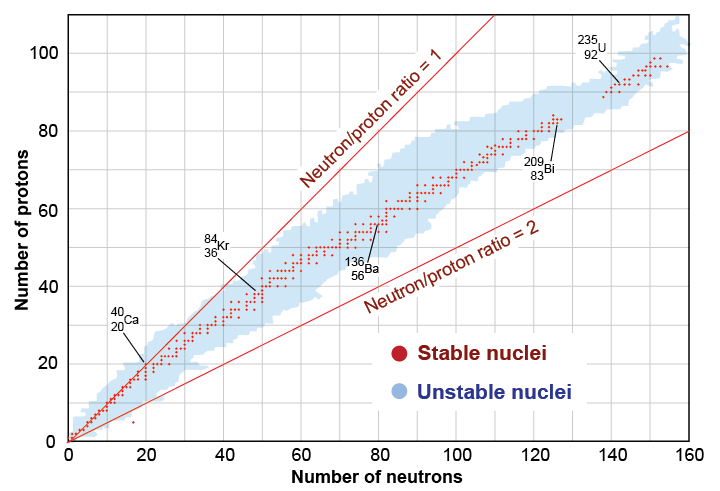
The balance of protons and neutrons is beautifully illustrated by graphing the number of protons versus the number of neutrons. The upper red line represents nuclei with equal numbers of protons and neutrons (N = Z). The red circles represent stable nuclei, such as carbon-12. The blue region on the graph shows nuclei, such as uranium-238, that may exist but are unstable (or radioactive). The white area represents combinations of neutrons and protons that cannot remain bound to form a nucleus, even for a nanosecond. 
|
Notice that stable nuclei tend to lie close to the line N = Z up to atomic number 20 (calcium). Past calcium, the stability line bends down, indicating more neutrons than protons. For example, uranium-235 has 92 protons and 143 neutrons, which is 51 more neutrons than protons. As the size of the nucleus increases, the short range of the strong force cannot bind the outermost nucleons as tightly as those near the center. To maintain equilibrium in large nuclei requires an excess of neutrons. The extra neutrons “dilute” the effect of the repulsive Coulomb force. 
|
| |
|

