|
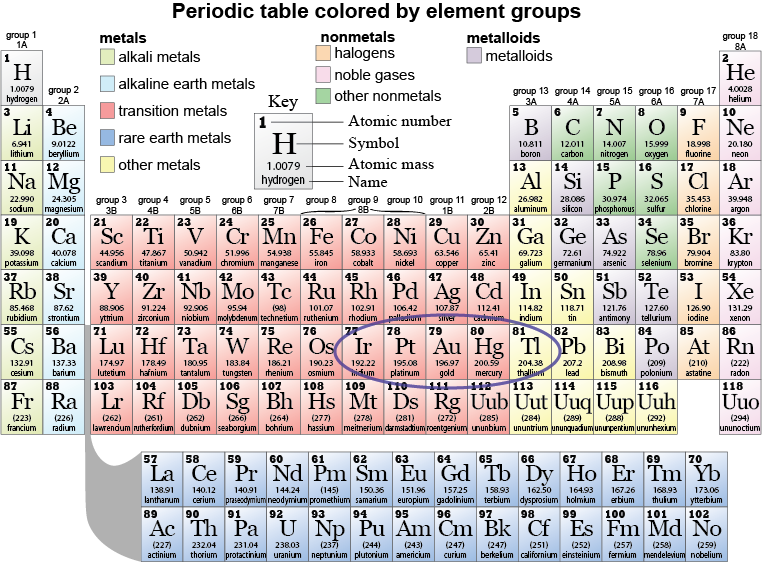
The periodic table is an indispensable tool in science. It is organized by the chemical behavior of the elements and it gives us a map for predicting the ability of elements to combine and form chemical compounds. The periodic table does not contain information about isotopes since isotopes of the same element have the same electron structure and therefore the same chemical properties. The only isotope-related information in the periodic table is contained in the atomic mass of the elements, which represents the average mass for all isotopes of that element. 
|
|
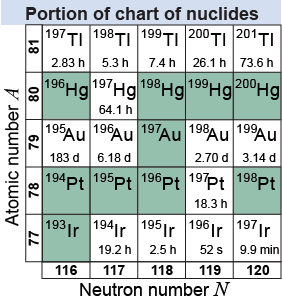 In nuclear science, nuclear engineering, nuclear medicine, and any other nuclear discipline, nuclides need to be organized according to their isotopes. The chart of nuclides is a table that maps the atomic number Z and the neutron number N for each element. The chart of nuclides also contains information about the radioactive properties of the nuclides. Here is an example of a portion of the chart of nuclides centered at gold . Note the various isotopes for gold. The row above gold gives information about the isotopes of mercury (Hg), which is to the right of Au on the periodic table. The row below Au gives information about the isotopes of platinum (Pt). The green squares represent stable isotopes. The time shown on the white squares is the half-life of the particular isotope—which we will learn about on page 801.
In nuclear science, nuclear engineering, nuclear medicine, and any other nuclear discipline, nuclides need to be organized according to their isotopes. The chart of nuclides is a table that maps the atomic number Z and the neutron number N for each element. The chart of nuclides also contains information about the radioactive properties of the nuclides. Here is an example of a portion of the chart of nuclides centered at gold . Note the various isotopes for gold. The row above gold gives information about the isotopes of mercury (Hg), which is to the right of Au on the periodic table. The row below Au gives information about the isotopes of platinum (Pt). The green squares represent stable isotopes. The time shown on the white squares is the half-life of the particular isotope—which we will learn about on page 801. 
|
| |
|

