|
The masses of the proton and neutron are so small (about 1.67 × 10−27 kg) that nuclear scientists often work in atomic mass units. One atomic mass unit (amu) (1 amu) is defined to be one-twelfth (1/12) the mass of the carbon-12 atom. Another way to state this is that the atomic mass of carbon-12 is defined as being exactly 12 amu. Why one-twelfth? Carbon-12 has six protons and six neutrons, so the atomic mass unit is about the average mass of a nucleon. 
|
| | Atomic mass unit (amu)
|
|
One atomic mass unit is 1.661 × 10−27 kg, which is slightly less than the mass of a single proton. The electron has a mass of 0.0005 amu, which means that the electron has around 0.05% of the mass of a nucleon. 
|
 The periodic table of the elements contains information about nuclear structure as well as chemical properties. The atomic number is the number of protons in the nucleus and the atomic mass is the average mass of one atom in atomic mass units. Note that the atomic mass given in a periodic table is the average mass, which includes all isotopes that naturally occur on Earth. The atomic mass for carbon, 12.011 amu, is not precisely 12.000 because naturally occurring carbon contains a small amount of the isotopes carbon-13 and carbon-14. The atomic mass also includes the mass of the electrons, which is very small compared to the mass of the nucleus.
The periodic table of the elements contains information about nuclear structure as well as chemical properties. The atomic number is the number of protons in the nucleus and the atomic mass is the average mass of one atom in atomic mass units. Note that the atomic mass given in a periodic table is the average mass, which includes all isotopes that naturally occur on Earth. The atomic mass for carbon, 12.011 amu, is not precisely 12.000 because naturally occurring carbon contains a small amount of the isotopes carbon-13 and carbon-14. The atomic mass also includes the mass of the electrons, which is very small compared to the mass of the nucleus. 
 |
The atomic mass is weighted by the natural abundance of each isotope. Carbon on Earth is 98.9% carbon-12, about 1.1% carbon-13, and only a trace amount of carbon-14. 
|
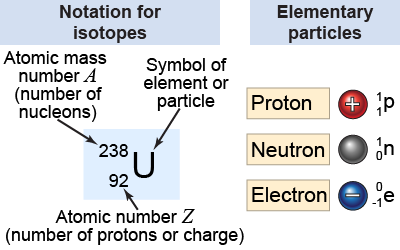 An isotope is identified by providing both the atomic number Z and the mass number A. The number of neutrons, N, in the isotope can be calculated by using the relationship A = N + Z. One way to write down an isotope uses the name of the element and the atomic mass number, such as “carbon-12.” Alternatively, a special notation is used that includes the abbreviation for the element, the atomic number, and the atomic mass number. A subscript to the left of the element name represents the atomic number Z. A superscript, also to the left of the element, represents the atomic mass number A. The general form of this special notation for any isotope of an element X is .
An isotope is identified by providing both the atomic number Z and the mass number A. The number of neutrons, N, in the isotope can be calculated by using the relationship A = N + Z. One way to write down an isotope uses the name of the element and the atomic mass number, such as “carbon-12.” Alternatively, a special notation is used that includes the abbreviation for the element, the atomic number, and the atomic mass number. A subscript to the left of the element name represents the atomic number Z. A superscript, also to the left of the element, represents the atomic mass number A. The general form of this special notation for any isotope of an element X is . 
|
For example, carbon-12 has A = 12 and Z = 6 and is denoted as . The isotope uranium-238 is written as . To determine the number of neutrons, N, we solve the equation A = N + Z, or 238 = 92 + N. The number of neutrons in uranium-238 is N = 238 − 92 = 146. 
|
The same notation is used to represent the elementary particles. The proton is because it has one nucleon and one positive elementary charge. The neutron is because it is neutral. The electron is because it is not a nucleon and has negative charge. 
|
The mass of one atom of a certain isotope of polonium (Po) is 3.471×10−25 kg. What isotope of polonium is this? - polonium-2
- polonium-84
- polonium-100
- polonium-209
 |
The correct answer is d. Most of an atom’s mass is in the nucleus, and the atomic mass unit is slightly less than the mass of a proton, so dividing the mass of the atom by the mass associated with 1 amu will tell us the number of nucleons: | |
This atom has 209 nucleons 
|

