|
Radioactive decay is the spontaneous emission of an energetic particle or photon from a nucleus. The three types of radioactive decay are called alpha, beta, and gamma. Radioactive materials are also identified by their half-life, the time in which half of the original atoms have decayed. Carbon dating is an effective method for dating the age of biological relics and is based on the 5,730 year half-life of carbon-14. (See page 797 for further references to research the historical development of the concept of the weak nuclear force.) 
|
|
radioactive decay, alpha decay, beta decay, gamma decay, weak nuclear force, half-life, carbon dating
|
|
|
|
Review problems and questions |
|
- Research and describe the historical development of the concept of the weak force.


|
Answers for this may vary, but key points in an essay should include the following:- The weak force was first proposed in the 1930s by Enrico Fermi,
- The weak force was needed to explain why radioactive decay occurs,
- A theory proposed in 1968 by Weinberg, Glashow, and Salam unified the weak force with the electromagnetic force at high energies,
- Experimental confirmation of that theory of the electroweak interaction occurred through observations of neutrino scattering and the discovery of two new particles.

|
- Uranium-235 undergoes beta decay according to this nuclear reaction equation:
| |
What is the element and isotope produced? 

|
Answer: neptunium-235
The atomic mass number must be the same on both sides of the nuclear reaction equation, so or A = 235. Equating the atomic number on both sides of the reaction equation gives or Z = 93. On the periodic table, atomic number 93 is Np (neptunium). The element and isotope produced is therefore 
|
- The unstable uranium isotope 235 undergoes radioactive decay to produce thorium-231 following the equation What is the atomic and mass number of the emitted particle? What is the name for this particle and type of decay?


|
Answer: The atomic number is Z = 2, and the atomic mass number is A = 4. This is an alpha particle (helium-4 nucleus) produced during alpha decay.
Equating the atomic mass number on both sides of the nuclear reaction equation gives or A = 4. Doing the same for the atomic number on both sides of the reaction equation gives or Z = 2. On the periodic table, atomic number 2 is He (helium). The particle produced is therefore This is an alpha particle, and the uranium has undergone alpha decay. 
|
- In the radioactive decay equations in questions #2 and 3 above, do you expect the binding energy of the resultant nucleus (or nuclei) to be larger or smaller than the binding energy of the original nucleus?

|
The binding energy of the resultant nucleus will be smaller than the original nucleus. The difference in energy is released, in particular as kinetic energy for the resulting nucleus and alpha or beta particle. 
|
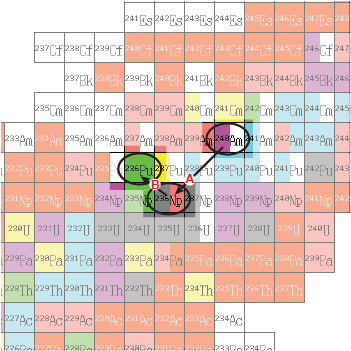
- A two level decay process starting with americium-240 ends up with plutonium-236, as indicated by the arrows on this relevant portion of the chart of nuclides. These two levels are indicated by the arrows marked A and B on the chart of nuclides.
What are these two decay processes? 

|
Answer: Process A is alpha decay; process B is beta decay.
Process A is alpha decay because Z and N each decrease by 2. The reaction is Process B is beta decay since Z increases by 1 and N decreases by 1. The mass number does not change. The reaction is 
|
Take a Quiz |

