|
| Essential questions | | How does convection transfer heat? | |
|
Convection transfers heat through the movement of matter. When matter such as air or water moves, the thermal energy contained in that matter also moves. Convection is the dominant mode of heat transfer in fluids such as air and water. 
|
Part 1: Observing convection
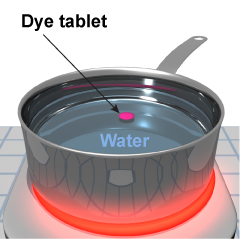
- Fill a pan with cold water and set it on a hot plate at high heat.
- Drop one tablet of egg dye into the pot and observe the motion of the colored water as the tablet dissolves.
- Turn off the heat and repeat the experiment with another tablet.
- What differences do you observe when the heat is on compared to when the heat is off?
- Calculate the equivalent power in heat transferred when 1 kg of water at 20ºC replaces 1 kg of water at 10ºC in 1 s.
- Calculate the power in heat transferred by conduction through a cubic container filled with 1 kg of water. One wall of the container is at 20ºC and the opposite wall is at 10ºC.
- How do the two heat transfer power values compare with each other? Which is greater? Are they much different or only a little different?

|
Part 2: Comparing convection and conduction
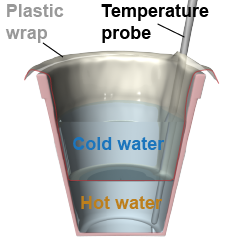
- Fill half a 20 oz foam cup with hot water and insert a temperature probe against one side.
- Loosely set a sheet of thin plastic wrap over the cup and start recording the temperature at a rate of once every 5 s.
- Pour some ice water into the plastic wrap so it can exchange heat with the hot water through the plastic wrap but is not allowed to mix. Take temperature data for 3 min.
- Repeat the experiment without the plastic wrap, allowing the hot and cold water to mix. Keep the data collection at the same rate so you can compare the two experiments.
- Create graphs of these two cooling curves using the same horizontal and vertical scales.
- Describe the difference in the rate of temperature change you observe when the water is allowed to mix compared to when it is prevented from mixing by the plastic wrap.
- Estimate the rate of heat transfer through the plastic wrap. Is the plastic wrap a factor in limiting the overall heat transfer by conduction? (The specific heat of water is 4,184 J/[kg °C].)
- Discuss the relative contributions of conduction and convection in this experiment.
- How did the distribution of energy between the hot and cold water change when you used the plastic wrap? How did it change when you removed the plastic wrap?

|

