|
 The thermal energy carried by conduction and convection is fundamentally the energy in random motion of atoms and molecules in matter. This is why conduction and convection only occur in matter. It also explains why heat moves relatively slowly in both conduction and convection. Energy must be ultimately transferred by trillions of tiny, random collisions between individual molecules. Conduction occurs in all phases of matter: solid, liquid, and gas. Convection occurs only in the liquid and gas phases in which entire collections of molecules can move as a whole in addition to their individual thermal motion.
The thermal energy carried by conduction and convection is fundamentally the energy in random motion of atoms and molecules in matter. This is why conduction and convection only occur in matter. It also explains why heat moves relatively slowly in both conduction and convection. Energy must be ultimately transferred by trillions of tiny, random collisions between individual molecules. Conduction occurs in all phases of matter: solid, liquid, and gas. Convection occurs only in the liquid and gas phases in which entire collections of molecules can move as a whole in addition to their individual thermal motion. 
|
 Radiation is fundamentally different from conduction and convection. Electromagnetic waves are pure energy independent of matter. This is why radiation can transfer heat through the vacuum of space. Radiation also moves at the speed of light, 3 × 108 m/s, far faster than other types of heat transfer. When radiation encounters matter, some of the electromagnetic energy is transformed into molecular thermal motion—ordinary heat.
Radiation is fundamentally different from conduction and convection. Electromagnetic waves are pure energy independent of matter. This is why radiation can transfer heat through the vacuum of space. Radiation also moves at the speed of light, 3 × 108 m/s, far faster than other types of heat transfer. When radiation encounters matter, some of the electromagnetic energy is transformed into molecular thermal motion—ordinary heat. 
|
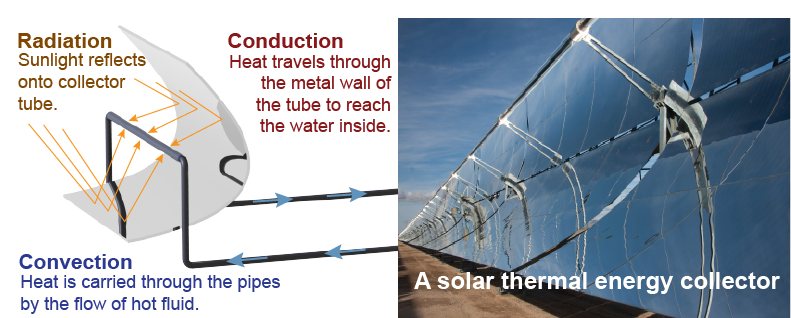
Our world of matter and energy includes solids, liquids, and gases at temperatures far above absolute zero. All three forms of heat transfer act together to determine the rate at which thermal energy is exchanged. Which of the three is most important depends on the temperature and on the circumstances. Although it is uncommon for all three modes of heat transfer to be equally important in a single transformation of energy, heat transfer usually occurs in many steps. In both nature and technology, a different form of heat transfer may be most important at each different step. For example, in a solar-thermal power station, radiant energy heats a metal tube. The heat travels through the walls of the tube by conduction, where it heats water into steam, which in turn carries the heat to a turbine by convection. 
 |
Heat flow is a critical factor in many aspects of technology. For example, computer chips generate heat from electrical resistance. Inside computers on top of the CPU and graphics chips are heat sinks that conduct heat away from the chip. On top of the heat sinks are fans that use forced convection to transfer the heat to air. If the heat is not conducted away fast enough, the microcircuitry inside malfunctions or melts. Materials with high thermal conductivity, including diamond, are used to carry heat away from high-performance computer chips. 
|
|
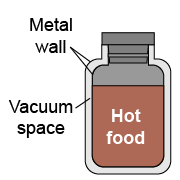 A Thermos® or vacuum bottle is an insulated container that keeps food warm for many hours by reducing heat transfer from the inside to the outside. Which is the best reason why Thermos® bottles have a vacuum space (no air) between the inner and outer walls?
A Thermos® or vacuum bottle is an insulated container that keeps food warm for many hours by reducing heat transfer from the inside to the outside. Which is the best reason why Thermos® bottles have a vacuum space (no air) between the inner and outer walls? - Radiation cannot transfer heat through a vacuum.
- Convection cannot transfer heat through a vacuum.
- Vacuum has no mass and is therefore lighter than other insulators such as foam.
 |
The correct choice is b because both convection and conduction act only through matter and a pure vacuum space prevents both from occurring. Of course, in a real Thermos® bottle the vacuum is not perfect so there is some very low pressure gas in this space, but the heat transfer by both conduction through the gas and convection is reduced more than a factor of 100 compared to having matter such as foam as an insulator. 
|
| |
|

