|
The electrostatic force exists between any two objects that have a nonzero net charge (positive or negative). The quantitative description of the electrostatic force is called Coulomb’s law and is given in equation (18.1). Coulomb’s law says the force between two charges is proportional to the product of the charges divided by the square of the distance between them. The unit of electric charge in Coulomb’s law is the coulomb (C). 
|
| (18.1) | | | Fe | = | electrostatic force (N) | | ke | = | Coulomb constant = 9.0×109 N m2 / C2 | | q1 | = | electric charge of object 1 (C) | | q2 | = | electric charge of object 2 (C) | | r | = | distance between the two objects (m) |
| Coulomb’s law
|
|
The Coulomb constant is ke = 9×109 N m2/C2, which means that the electric force is very strong. There are about 2,000 C of positive and negative charges in one drop of water. If you could hold these charges one meter apart the attractive force between them would be 40,000,000,000,000,000 N (4×1016 N)! Because the coulomb force is so strong, on the rare occasions that separated charges do occur, such as static electricity, the amount of excess positive or negative charge is extremely small. Most applications in physics involve charges of microcoulombs. One microcoulomb (μC) is one-millionth of a coulomb, or 10−6 C. 
 |
The unit of electric current is the ampere, which is equivalent to the flow of one coulomb of charge past a point in a circuit every second. You can be hurt physically by the flow of just 0.1 A through your body, so that provides an indication how much charge an entire coulomb represents! Even though large amounts of charge can flow in an electric current, the wire itself still contains equal amounts of positive and negative charges. 
|
The Coulomb force follows an inverse square law, similar to the gravitational force. If two charged bodies are moved further apart by twice their distance, their mutual electric force is reduced by a factor of 1/(22) = ¼. 
|
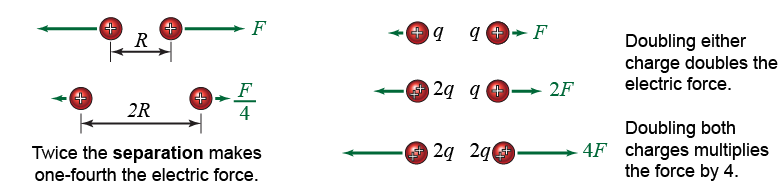
|
The electric force between two charged objects depends on the product of the two charges. If charge q1 doubles then the electric force also doubles. If charge q2 also doubles then the force doubles again and is four times greater. If one of the two objects has zero net charge, then either q1 or q2 is zero and the force in equation (18.1) is zero. 
|
Two electric charges of +1 μC and −1 μC are separated by 1 cm. What is the strength and direction of the force between them? | Asked: | the force Fe | | Given: | q1 = q2 = 1×10−6 C; r = 1 cm = 0.01 m | | Relationships: | | | Solution: | | | Answer: | The force is 90 N and is attractive since the charges have opposite polarity. | 
|
If two 10 μC negatively charged objects experience a force between them of 22.5 N, how far apart are they? - 0.0400 m
- 0.200 m
- 0.474 m
- 63.0 m
 |
Asked: distance r between two objects
Given: objects of charge q = 10 μC = 10−5 C; objects experience force F = 22.5 N;
Coulomb’s constant ke = 9.0×109 N m2/C2
Relationships: Solve: Rearrange this equation to solve for r: 
|

