|
By 1908 it was known that the atom contained positive and negative charges. One of the big unknowns was what the charges were. How could one measure the charge e on a single electron? The value of the elementary charge e is a fundamental quantity of physics, and its measurement was a challenge for experimenters of the day. The crucial experiments were done between 1908 and 1913 by the American physicist Robert Millikan at the University of Chicago. Working with his graduate student Harvey Fletcher, Millikan undertook a grueling series of very sensitive experiments that determined the charge on the electron to be approximately 1.59×10−19 C. This is within 0.6% of its modern value of 1.602×10−19 C. 
|
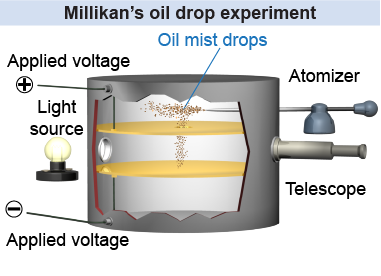 In Millikan’s experiment very tiny oil mist droplets are allowed to fall between two charged metal plates. Friction from an atomizer nozzle leaves each droplet with a small static charge. The voltage between the plates can be adjusted from 0 to >1,000 V, creating a uniform electric field between the plates. The electric field is adjusted until a droplet is suspended in equilibrium by balancing its weight against the electric force.
In Millikan’s experiment very tiny oil mist droplets are allowed to fall between two charged metal plates. Friction from an atomizer nozzle leaves each droplet with a small static charge. The voltage between the plates can be adjusted from 0 to >1,000 V, creating a uniform electric field between the plates. The electric field is adjusted until a droplet is suspended in equilibrium by balancing its weight against the electric force. 
|
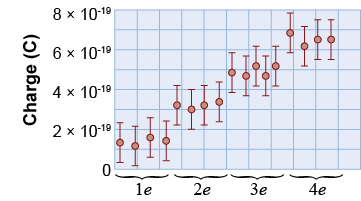 Using a telescope, Millikan adjusted the voltage until only a single drop was in sight. By carefully raising and lowering the voltage, and measuring the velocity of the oil drop, Millikan was able to determine the charge on one drop to a very precise value. His data showed that the charge on an oil drop always occurred in multiples of 1.59×10−19 C!
Using a telescope, Millikan adjusted the voltage until only a single drop was in sight. By carefully raising and lowering the voltage, and measuring the velocity of the oil drop, Millikan was able to determine the charge on one drop to a very precise value. His data showed that the charge on an oil drop always occurred in multiples of 1.59×10−19 C! 
|
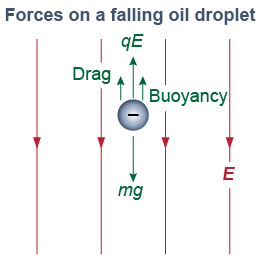 The plate voltage provided Millikan with a very accurate measure of the electric field. To determine the charge, however, he also needed to know the mass of the oil droplet. To determine the mass, Millikan measured the terminal velocity as each droplet fell at constant speed. When falling at its terminal velocity, the weight of the oil drop is balanced against the air resistance, buoyancy, and electric forces combined. In a more advanced course on fluid dynamics, you may learn that the drag force F on a sphere of radius r moving through a liquid is 6πrηv, where v is the velocity and η is the viscosity of the fluid (or air in this case). Once he had the radius, Millikan could work out the mass using the density of the oil. Millikan’s early value for e is lower than the modern value mostly because he underestimated the viscosity of air.
The plate voltage provided Millikan with a very accurate measure of the electric field. To determine the charge, however, he also needed to know the mass of the oil droplet. To determine the mass, Millikan measured the terminal velocity as each droplet fell at constant speed. When falling at its terminal velocity, the weight of the oil drop is balanced against the air resistance, buoyancy, and electric forces combined. In a more advanced course on fluid dynamics, you may learn that the drag force F on a sphere of radius r moving through a liquid is 6πrηv, where v is the velocity and η is the viscosity of the fluid (or air in this case). Once he had the radius, Millikan could work out the mass using the density of the oil. Millikan’s early value for e is lower than the modern value mostly because he underestimated the viscosity of air. 
|
| |
|

