|
Matter is made of atoms and electric charge is a property of the particles inside every atom. That means that on a very fundamental level, similar to mass, electric charge is a basic property of matter. Mass can only be zero or positive. (Even antimatter has positive mass!) Electric charge, however, can be positive, negative, or zero (neutral). 
|
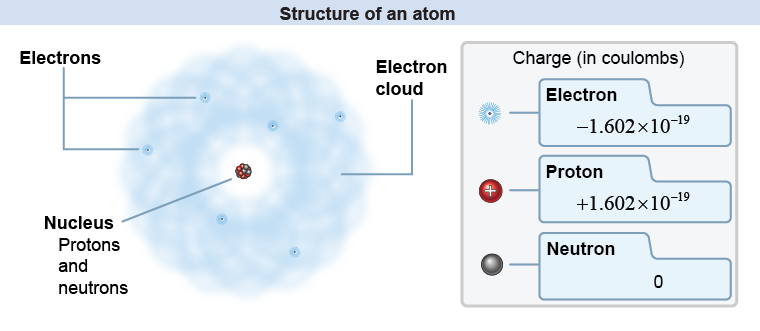
The nucleus of the atom contains positive charged particles called protons and neutral particles called neutrons. Outside the nucleus are tiny, fast-moving negative charged particles called electrons. The negative electrons are attracted to the positive protons in the nucleus and that is what holds atoms together. The electrons farthest from the nucleus of one atom may also be attracted to the nucleus of another atom, and the resultant sharing of electrons between two atoms is the underlying explanation for chemical bonds that group atoms into molecules and compounds. In fact, most of the behavior of matter—including static electricity and most of chemistry—comes from the activity of electrons. 
|
|
The charge on the proton and electron are exactly equal and opposite. A complete atom has an equal number of protons and electrons; therefore, a whole atom has zero net electric charge. Chapter 26 covers the atom in more detail; to understand electricity in this chapter, it is important to know that matter is made of atoms and that electric charge is a property of the protons and electrons in all atoms. 
|
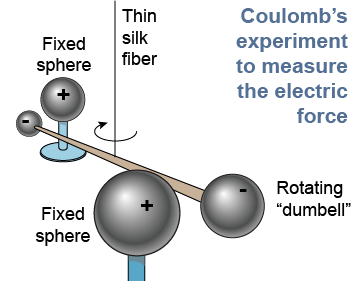 The unit of electric charge is the coulomb (C) in honor of Charles-Augustin de Coulomb (1736–1806). A French physicist, Coulomb made the first accurate measurements of the electrical force between charges. A coulomb is a very large amount of charge! One coulomb of charge is equal to the charge of 6×1018 electrons. Ordinary static electricity results from an excess charge of less than one-millionth of a coulomb.
The unit of electric charge is the coulomb (C) in honor of Charles-Augustin de Coulomb (1736–1806). A French physicist, Coulomb made the first accurate measurements of the electrical force between charges. A coulomb is a very large amount of charge! One coulomb of charge is equal to the charge of 6×1018 electrons. Ordinary static electricity results from an excess charge of less than one-millionth of a coulomb. 
|
A carbon-13 atom has six protons and seven neutrons in its nucleus. How many electrons does the atom have?
 |
“Complete” atoms are electrically neutral; therefore, they have equal numbers of protons and electrons. Since the carbon-13 atom has six protons in its nucleus, the atom also has six electrons. 
|

